Draw the products of benzoic acid reacting with sodium hydroxide. Draw the products of pyridine reacting with hydrochloric acid.

Draw The Products Of Benzoic Acid Reacting With Sodium Hydroxide Draw The Products Of The Pyridine Reacting With Hydrochloric Acid Use The Button To Add The Charge And H Atom
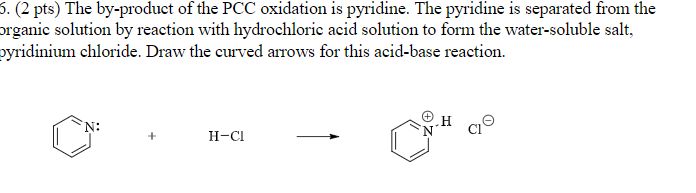
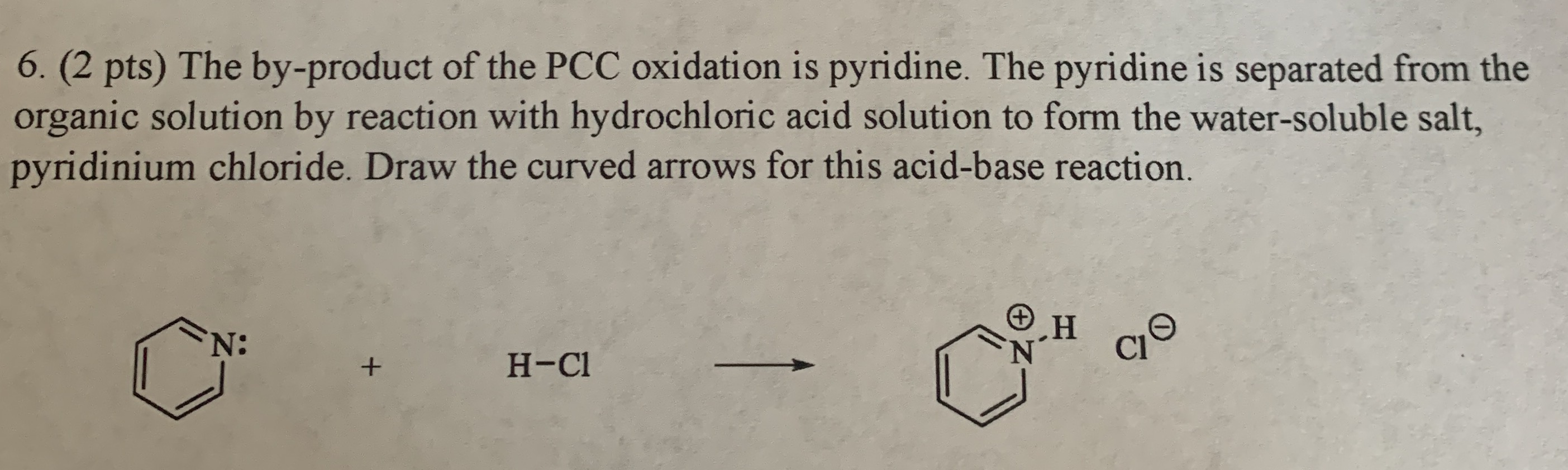
Draw the products of pyridine reacting with hydrochloric acid.
. NEW X FG CI Br 1 P x х JSME Molecular Editor by Peter Ertl and Bruno Bienfait 3. C6H5COOH NaOH C6H5COO-Na H2O. Science Chemistry QA Library For the reaction between ammonia NH3 and hydrochloric acid HCI a draw out the Lewis structures for the reactants given b identify the Lewis acid and Lewis base c draw the curved arrows that show the direction of electron flow to form the product d draw the structure of the product s you would expect.
Rep Click the draw structure button to launch the drawing utility. These reactions are characterized by the presence of one proton. Properties of benzoic acid.
Usually aqueous sodium hydroxide is used as the base catalyst but pyridine also can be used in this reaction. Your score for the pre-lab is. Acid-base reactions also called acid-base neutralization.
Reacts exothermically with organic bases amines amides and inorganic bases oxides and hydroxides of metals. 1500 out In your lab notebook you should also complete the written portion of and plan of procedure. We review their content and use your feedback to keep the quality high.
N H Cl. CH3SO2CI pyridine OH 2. Мaxwell presented by Macmillan Learning Predict the organic products of the reaction.
The pyridine is removed from the organic layer by washing with hydrochloric acid solution. CH3 O SOCI2 pyridine draw structure OH Next 4 of. HYDROCHLORIC ACID is an aqueous solution of hydrogen chloride an acidic gas.
Show a detailed mechanism for this reaction. The reaction is believed to involve initial attack by sulfur at nitrogen followed by nucleophilic addition of a second pyridine at C -4. Be sure to include stereochemistry in your products.
A crystalline product obtained from an attempted acetylation with pyridine and acetic anhydride in the presence of a niacytin hydrolysate is shown to be -acetyl-12-dihydro-2-pyridylacetic acid 1 produced by combination of the pyridine and the acetic anhydride. Draw the products of the pyridine reacting with hydrochloric acid. Draw the 3R4S product.
Draw the products of the pyridine reacting with hydrochloric acid. Include all intermediates but not transition states. 11.
The by-product of the PCC oxidation is pyridine. PCC HOlu NaCr07 H2SO4 C CH33 1. If we add HCl into this solution sodium benzoate will be converted back into benzoic acid.
Draw the major products of the following reactions if there is a reaction. Who are the experts. Draw the products of the pyridine reacting with hydrochloric acid.
Pyridine is an organic compound having molecular formula and hydrogen chloride has the chemical formula In structure of pyridine nitrogen is attached with one of the carbons present in the benzene ring. When pyridine is treated with thionyl chloride a synthetically useful dichloride salt is formed which can for example be transformed into pyridine-4-sulfonic acid. Most chemical properties of pyridine are typical of a heteroaromatic compound.
Draw the products of benzoic acid reacting with sodium hydroxide. Chemical reaction with physical states. Explain how this acid-base extraction effectively removes the pyridine from the organic layer.
New X O FG С N O F CI Br 1. Draw the major product of the following reactions. Now when pyridine reacts with hydrogen chloride results in the formation of pyridinium chloride.
In this reaction hydrogen form bond with nitrogen due to the lone pair. Experts are tested by Chegg as specialists in their subject area. Hydrochloric acid is a strong acid and dissociates as H and Cl-.
Reacts exothermically with carbonates including limestone and building materials containing limestone and hydrogen carbonates to generate carbon dioxide. 1 OsOд рyridine 2 products 2 NaSO3 or NaHSO3 in H20 Draw the 3S4R product. C6H5COOH s NaOH aq C6H5COO-Na aq H2O l Sodium benzoate is very polar ionic compound and soluble in water.
In organic reactions pyridine behaves both as a tertiary amine undergoing protonation alkylation acylation and N-oxidation at the nitrogen atom. Draw the skeletal structure of the principal organic product for the reaction below. Also use curly arrows to show the flow of electrons for each step.
Use the - button to add the charge and H atom. Draw the curved arrows for this proton transfer reaction and draw the resulting product. Now when pyridine reacts with HCl.
It is weakly basic and with hydrochloric acid it forms a crystalline hydrochloride salt that melts at 145147 C. The base also neutralizes the hydrochloric acid which is formed in the process thereby preventing the further protonation of the amide product formed. Draw all the products including byproducts for this reaction.

Draw The Products Of The Pyridine Reacting With En Ya Guru
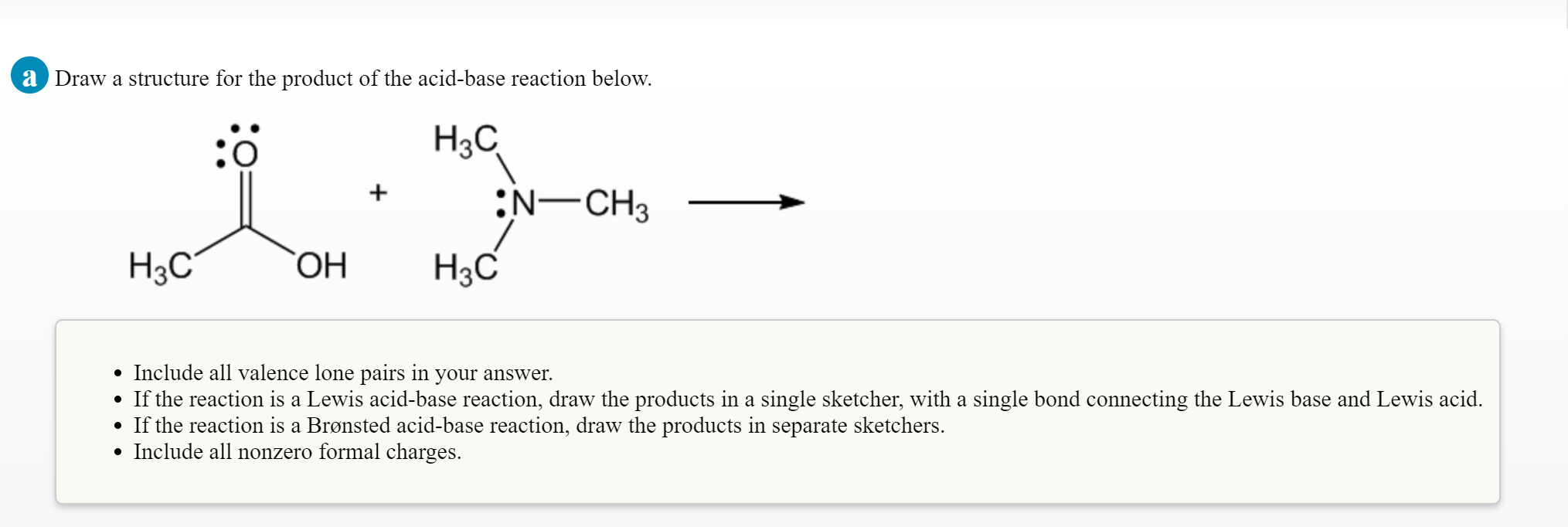
Solved Please Explain Additionally Can You Show The Flow Chegg Com
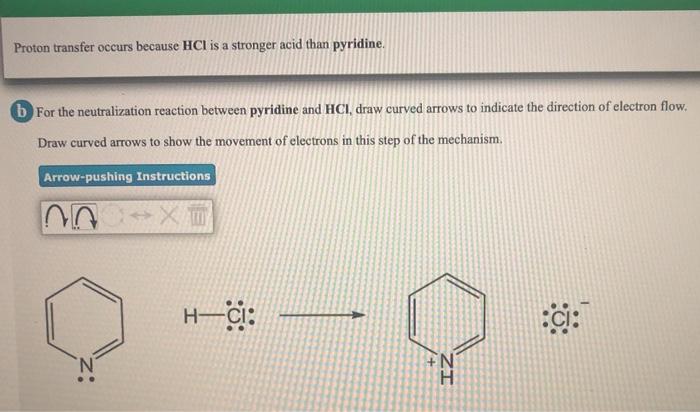
Solved Proton Transfer Occurs Because Hcl Is A Stronger Acid Chegg Com
Solved Pts Predict The Major Organic Product Of The Chegg Com
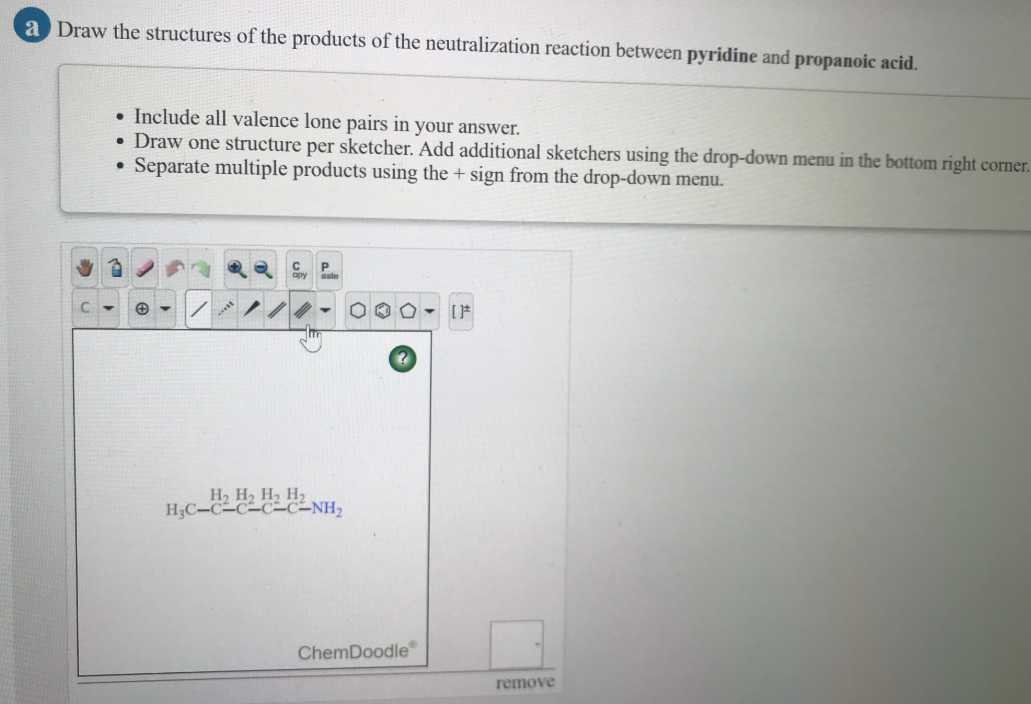
Solved A Draw The Structures Of The Products Of The Chegg Com

Solved 1 2 Pts Identify The Product Of The Chromic Acid Chegg Com
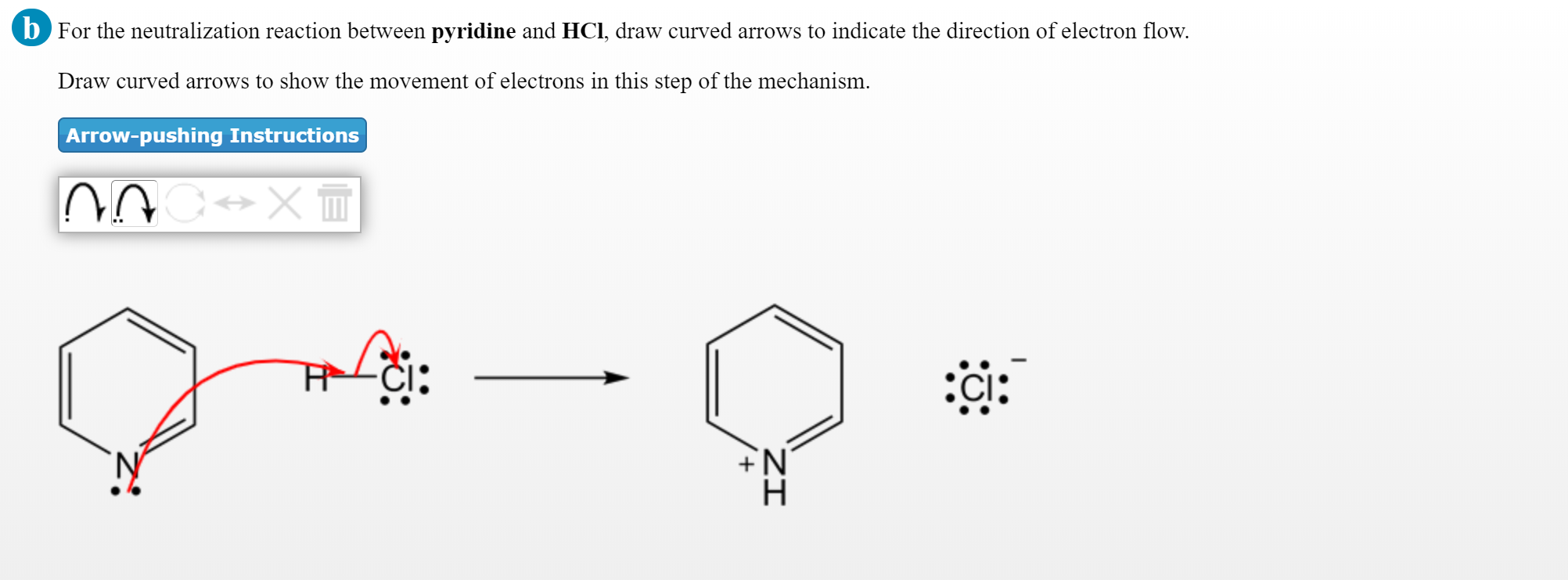
Solved Please Explain Additionally Can You Show The Flow Chegg Com

Oneclass What Are The Products Of The Pyridine Reacting With Hydrochloric Acid
0 comments
Post a Comment